
Artificial Intelligence and Robotics
A degree progamme for robot fans, AI enthusiasts and technicians.

A degree progamme for robot fans, AI enthusiasts and technicians.

Business Law - a combination with future!

The Computer Science degree programme is particularly well placed in rankings. It features four exciting specialisations and a flexible course of study - you can study AIN part-time, in combination with an apprenticeship or bilingually!

Our engineers receive modern and work-relevant training.

Our Advanced Precision Engineering master's degree programme focuses on pioneering fields such as lightweight construction and robotics. Become a specialist in innovative mechanical engineering with us!

Topics ranging from genetics to environmental sciences - ANB is particularly versatile

This programme deals with assistive technologies and how they are used to promote health.
The Applied Health Sciences degree programme deals with demographic change.

New materials for health, energy, mobility and communication - a key role in future technologies.

Decide for a study programme that offers you a wide range of job prospects
Start your career with the English-speaking medical engineering programme!

Three semesters of business management, marketing and sales

With our Business Administration and Engineering - Marketing and Sales degree programme, you will learn to apply the whole range of marketing tools and sales strategies.

If you are passionate about technology and innovation, you've come to the right place!

Complex IT systems are designed like architecture - this study programme shows you how!

Organising business processes well is the core of every successful business model. Our master's programme in Business Consulting gives you the tools to do just that!

In this new degree programme, you will learn to manage big data, improve business processes and make the most effective decisions for companies with the help of AI.

Whether modelling or optimising, you will become an expert in digital processes!

Business Management and Psychology - a business degree combination with a future!

Computer science with individual area of specialisation. The choice is yours!

Master's with 2 areas of specialisation: Software Engineering and Distributed Systems

An innovative degree programme with applied computer science media technology and design!
We will make you an interdisciplinary professional in modern media production!
With ELA you'll find innovative technical solutions for the future.

Our engineering psychology course combines technology with psychology. We make you an expert in user experience.

Strategic decisions and problem solving in complex professional situations

Don't just play games, create them yourself - Games & Immersive Media at HFU!

Improve your German language skills to CEF level C1 and prepare yourself for studying.

Using knowledge about usability and ergonomics for better products - Human Factors!

Unique combination of computer science and management. Optional: online study model!

You will study for one year at HFU, in Switzerland and in Alsace!

Inventing and playing with ideas is what it's all about - we're passionate about innovation!
The staging of interactive media in real and virtual spaces...

Whether individual modules, a "DAS" certificate or the entire master's degree - you decide!

Do you want to combine foreign languages and time spent abroad with business studies?

Acquire, store and process data and information digitally worldwide.

Traditional business studies, international orientation and language training

We have developed this MBA programme especially for experienced professionals!

Research, management practice and new ideas - in groups of around 15 students.

MMB - Advancing solutions and shaping the future!

MES - a specialist area with a high degree of innovation for your ideas!

How factories will be networked, processes automated & systems intelligent in the future

Media communication and design - that's where you come in!

Expertise in the approval of medical products - full-time or part-time!
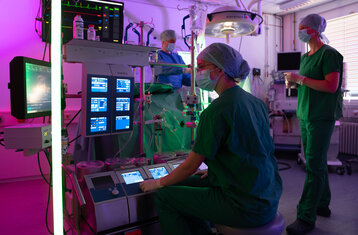
MKT Bachelor - health-oriented and patient-centred!

Development, realisation and approval of medical instruments & devices

Know-how in micromedicine, technology, biomedicine and biocompatible materials

Vocational qualification AND Bachelor of Science - Care of pregnant women and babies

Unique in Germany - study digital mobility!

How do diseases develop? How does an organism work? We shed light on medicine!

For this degree programme you need above all - creativity and talent!

Plan and control the use of online media - whether in industry or independently.
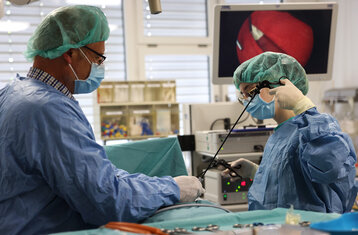
Working at the interface between medical and nursing staff

Your physiotherapy degree programme - direct help, with a double degree

Thanks to the double qualification (state examination and bachelor's degree), you have the academic background to back up your practical knowledge when helping patients.

Interface between intelligent machines/systems and production technologies

The interface of medicine, natural sciences and technology - multidisciplinary!
Pursue a career in safety and security with the master's degree programme in Risk, Reliability and Safety Engineering! We teach you everything you need to know about risk analysis and assessment.

Your study programme - protecting people, companies and the environment from danger!

We specialise in smart devices that can make "their own" decisions.

With us you can study and do an apprenticeship at the same time.

We train experts in environmentally friendly solutions.

Tuttlingen Campus - just try out which degree programme suits you best!
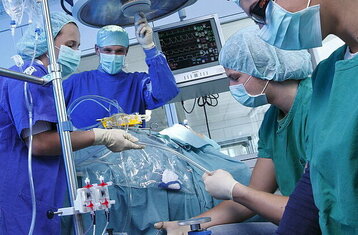
Our master's focuses on the interface between medicine and engineering.

The bachelor's degree can be taken with previous technical vocational qualification.

Sound knowledge of the global economy and intercultural competence

In this master's degree programme, you will build up your expertise in global business.

Skills in international management and marketing - outstanding career opportunities!
Study level
Location
Teaching language
Status
Give us your feedback
© Furtwangen University